Partner Institutions:

Institut national de la santé et de la recherche médicale, INSERM (UMR 1058), Montpellier, France

University of Bergen, Norway
 Centre Hospitalier Universitaire de Nantes, France
Centre Hospitalier Universitaire de Nantes, France
 Institut de Recherche pour le Développement (IRD), France
Institut de Recherche pour le Développement (IRD), France
 Médecins Sans Frontières –Epicentre, France
Médecins Sans Frontières –Epicentre, France
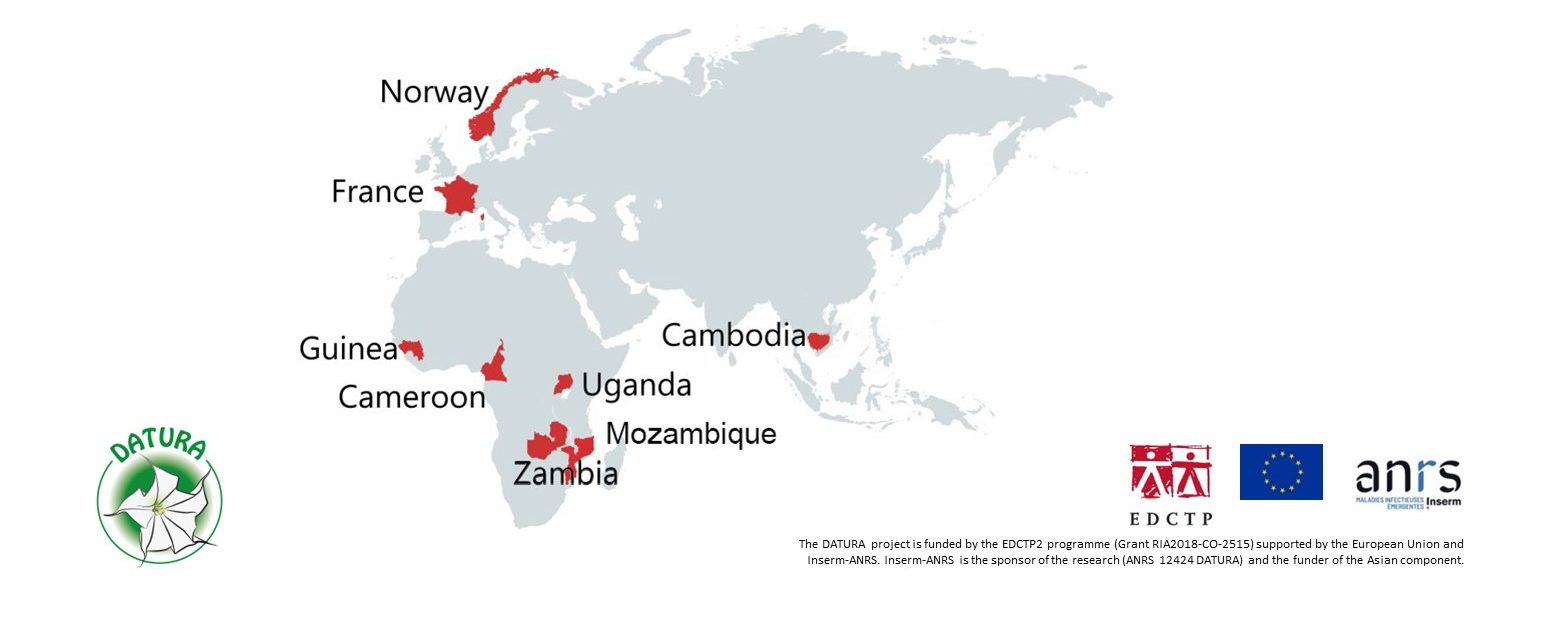
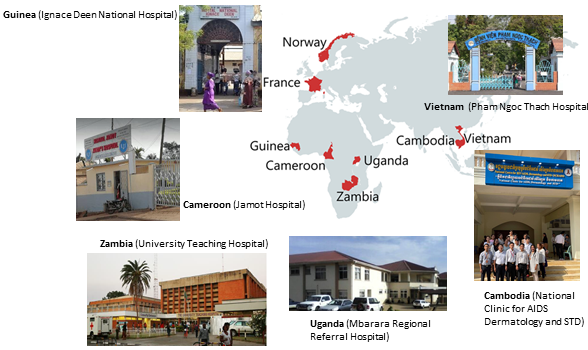
Hôpital national Ignace Deen, Conakry, GUINEA
 Central Hospital of Yaoundé, CAMEROON
Central Hospital of Yaoundé, CAMEROON
 University Teaching Hospital, Lusaka, ZAMBIA
University Teaching Hospital, Lusaka, ZAMBIA

Mbarara University of Science and Technology, UGANDA
 Institut Pasteur du Cambodge, Phnom Penh, CAMBODGE
Institut Pasteur du Cambodge, Phnom Penh, CAMBODGE
 National Centre for HIV/AIDS Dermatology and STDs, Phnom Penh, CAMBODIA
National Centre for HIV/AIDS Dermatology and STDs, Phnom Penh, CAMBODIA
Instituto Nacional de Saúde, Maputo, Mozambique