Hypothesis
We hypothesise that intensification of the initial phase of TB treatment, by increasing doses of two major TB drugs, rifampicin and isoniazid, and adding systematic corticosteroids, will decrease mortality in severely immunosuppressed HIV-infected adults and adolescents hospitalised for TB in comparison with the current standard TB regimen.
Objectives
Primary objective: To estimate the impact of an intensified initial phase of tuberculosis (TB) treatment on mortality at 48 weeks among HIV-infected adults and adolescents hospitalised for TB with CD4 ≤ 100 cells/μL in comparison with the standard TB regimen.
Secondary objectives: To estimate the impact of an intensified initial phase of TB treatment, in comparison with the standard TB regimen, on:
- Mortality at weeks 8 and 24
- Adverse events, including:
- All grade 3-4 events
- Selected grade 2 events of interest
- Drug-related adverse events
- AIDS defining illnesses
- Paradoxical TB-associated immune reconstitution inflammatory syndrome (IRIS)
- TB treatment success
- TB recurrence
- Antiretroviral treatment (ART) response in terms of virological success and immunological response
- Adherence to TB treatment and ART
- Full pharmacokinetics of rifampicin and isoniazid (and its N-acetyl-metabolite) at week 2
- Peak plasma concentrations of rifampicin and isoniazid (and its N-acetyl-metabolite) at day 3, day 7 and week 2
- Plasma concentrations of efavirenz and dolutegravir at week 4 (i.e. 2 weeks after the onset of ART)
Study design
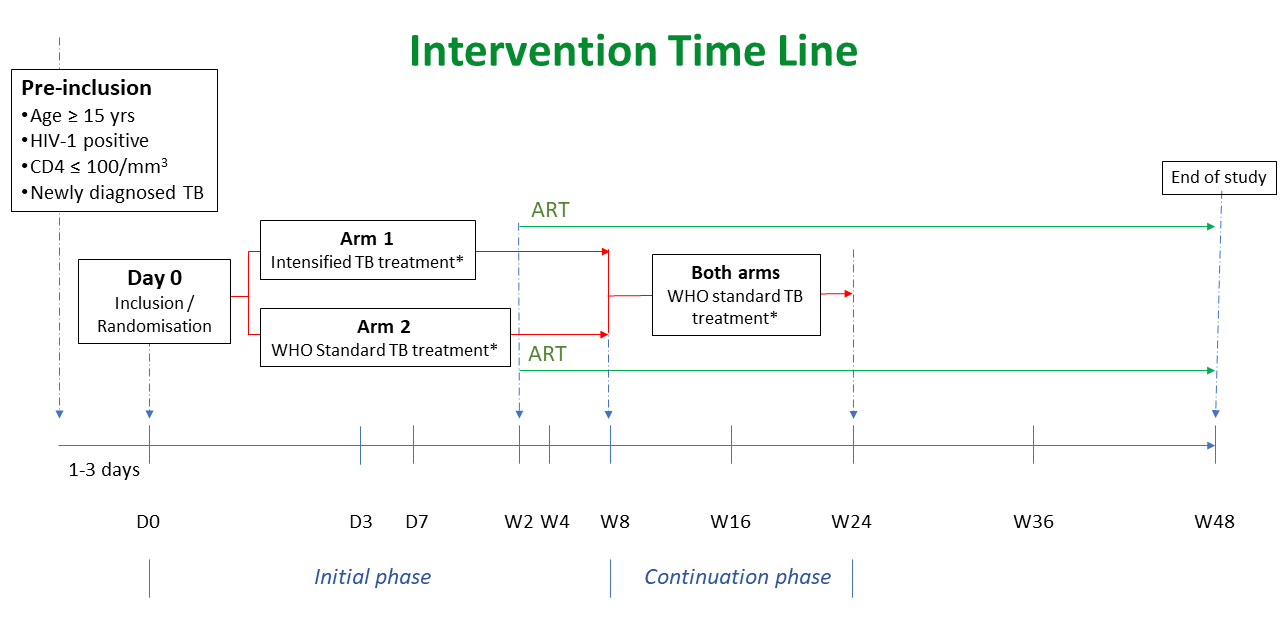
The DATURA trial is a phase III, multi-center, two-arm, open-label, randomised controlled superiority trial to compare the efficacy and the safety of two different TB regimens during the initial phase of treatment in HIV-infected adults and adolescents hospitalised for TB with CD4 ≤ 100 cells/μL over 48 weeks: intensified TB treatment and standard TB treatment.
It will be conducted in Cambodia, Cameroon, Guinea, Uganda, Mozambique and Zambia.
DATURA is registered in ClinicalTrials.gov : NCT04738812