DATURA is registered in ClinicalTrials.gov : NCT04738812
At inclusion, consecutive HIV-1 infected adults and adolescents with CD4 count ≤ 100 cells/μL hospitalised for newly diagnosed TB disease and not currently treated with antiretroviral treatment will be randomised in a 1:1 ratio to either:
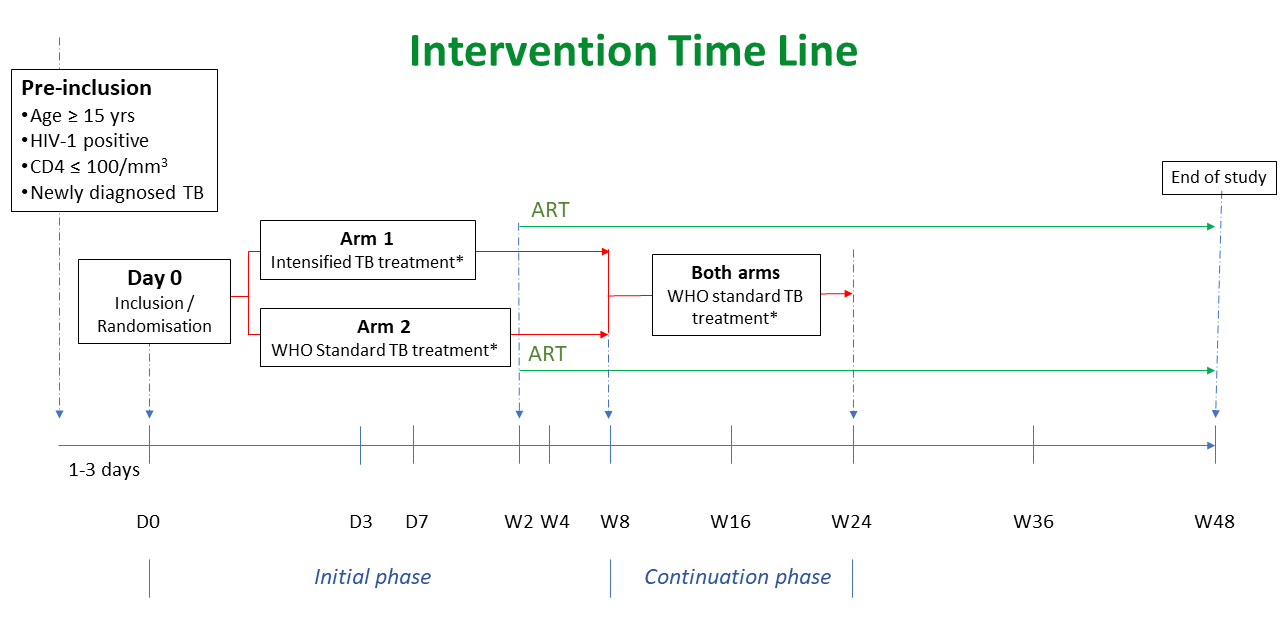
- Intensified TB treatment arm (ARM 1 – intervention arm):
- Increased doses of rifampicin (RIF) to 35±5 mg/kg daily and isoniazid (INH) 10±2 mg/kg daily together with standard-dose of pyrazinamide (Z) 20-30 mg/kg daily + ethambutol (E) 15-20 mg/kg daily for 8 weeks (initial phase of TB treatment),
- Adjunction of corticosteroid treatment for 6 weeks: Prednisone 40 to 80 mg once a day (OD) according to weight bands for 2 weeks, followed by 20 to 40 mg OD according to weight bands for 2 weeks, then 10 to 20 mg OD according to weight bands for the last 2 weeks (total duration: 6 weeks).
- Adjunction of albendazole 400 mg once a day for 3 days.
- WHO standard TB treatment arm (ARM 2 – control arm): Standard-dose rifampicin 10 mg/kg (8-12 mg/kg) daily + isoniazid 5 mg/kg (4-6 mg/kg) daily + pyrazinamide 25 mg/kg (20-30 mg/kg) daily + ethambutol 15 mg/kg (15-20 mg/kg) daily for 8 weeks (initial phase of TB treatment).