DATURA is registered in ClinicalTrials.gov : NCT04738812
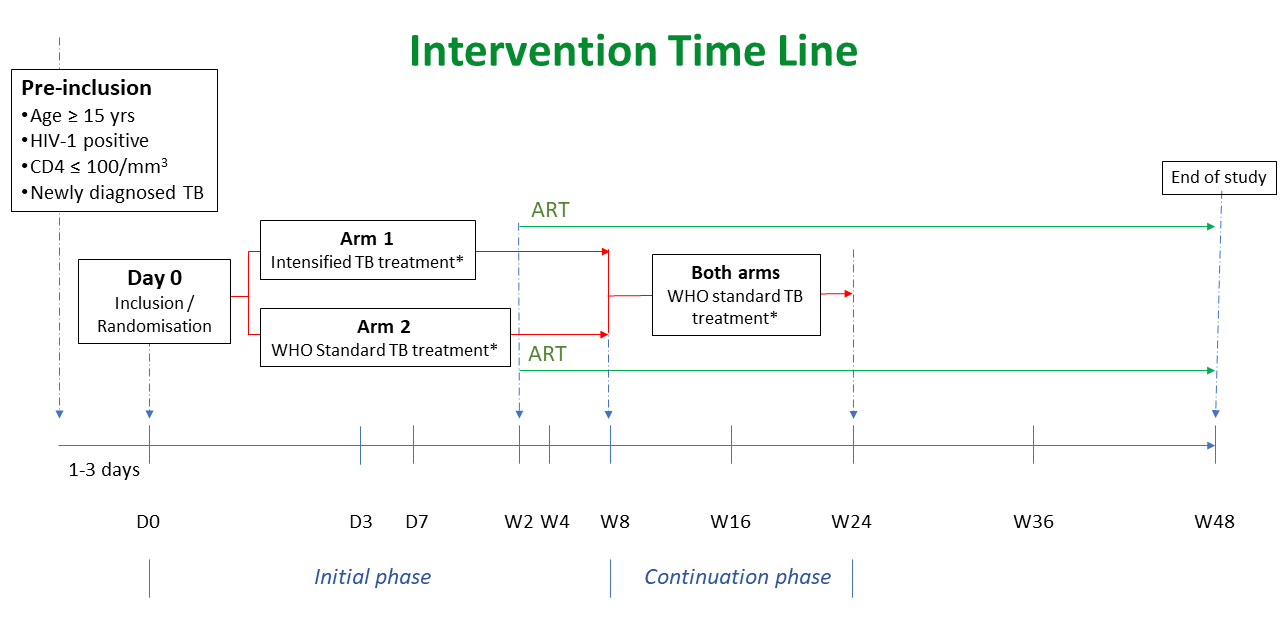
HIV-1 infected adults and adolescents hospitalised with active TB disease and not currently treated with ART for more than one week will be included in the trial and randomised between 2 arms.
Initial phase:
- ARM 1 – the intensified TB treatment arm (increased doses of rifampicin and isoniazid together with standard-dose of pyrazinamide and ethambutol for 8 weeks in addition to prednisone for 6 weeks)
- ARM 2 – the WHO standard TB treatment for 8 weeks.
The continuation phase of TB treatment will be identical in the two arms: 4 months of rifampicin and isoniazid at standard doses.
Patients will be followed-up for an additional 24 weeks (6 months) in the follow-up phase.
Learn more: